CURRENT SCENARIO OF DRUG RESISTANCE
The rampant rise of resistance to antibiotics is one of the biggest perils to global health today. Persistent use of antibiotics, self-diagnosis, and medication, exposure to infections in the hospitals, and misuse of antibiotics have all led to the emergence of multiple drug-resistant bacteria. Since the infections caused by these bacteria are hard to treat due to their resistance against most available antibiotics, they are now termed as ‘superbugs’. Some examples of such superbugs existing today include but are not limited to, Methicillin-resistant Staphylococcus aureus (MRSA) and Vancomycin-resistant Staphylococcus aureus (VRSA), both of which can cause sickness ranging from skin to invasive illnesses such as pneumonia and septicemia, multidrug-resistant Pseudomonas aeruginosa, a hospital-acquired pathogen that causes blood and lung infections, multidrug-resistant Acinetobacter which affects the urinary tract, lungs, blood and are present in wounds and carbapenem-resistant Enterobacteriaceae (CRE) which is also an invasive contagion. India is home to some of the biggest superbugs and researchers have estimated that by 2050, two million deaths are projected to occur due to antibiotic resistance. Therefore, there is an obvious and pressing need to address this situation.
USE OF PREDATORY BACTERIA
Since conventional therapies are now not very effective against multidrug resistance, research is now focused on exploring safe alternative approaches for combating this difficulty. A novel, developing area of study in this regard is the use of predatory bacteria as a potential therapy for fighting superbugs, by the study of predator-prey interactions. Bacterial predators make use of other pathogens as food by preying on them, thereby eliminating them. Some common examples of predatory bacteria are Myxococcus xanthus, Bdellovibrio bacteriovorus and Micavibrio species. Lately, studies pertaining to the use of Bdellovibrio bacteriovorus as a ‘living antibiotic’ is enthusiastically being carried out, for the reason being this obligate predator, although harmful to other pathogens in the microbial world, is absolutely harmless to humans. The lifecycle of this bacteria is primarily divided into two main phases: a free-living, non-replicative and rapidly swimming attack phase and an actively dividing, non-motile growth phase. The bacteria feeds by first latching itself to the surface of its prey and forming a bdelloplast (a modified form of Bdellovibrio bacteriovorus developed inside its host) post its entry. A gradual elongation of the bdelloplast inside the pathogen occurs due to consumption of its host as food, followed by its septation and lysis of the pathogen to reveal multiple re-established Bdellovibrio. These are then ready to hunt for its next pathogen/prey. A unique attribute that makes this predator a passionate subject for study is that it feeds without competition and reduces the population of prey drastically, therefore making it an ideal prospective candidate for use in therapy and as biocontrol agents.
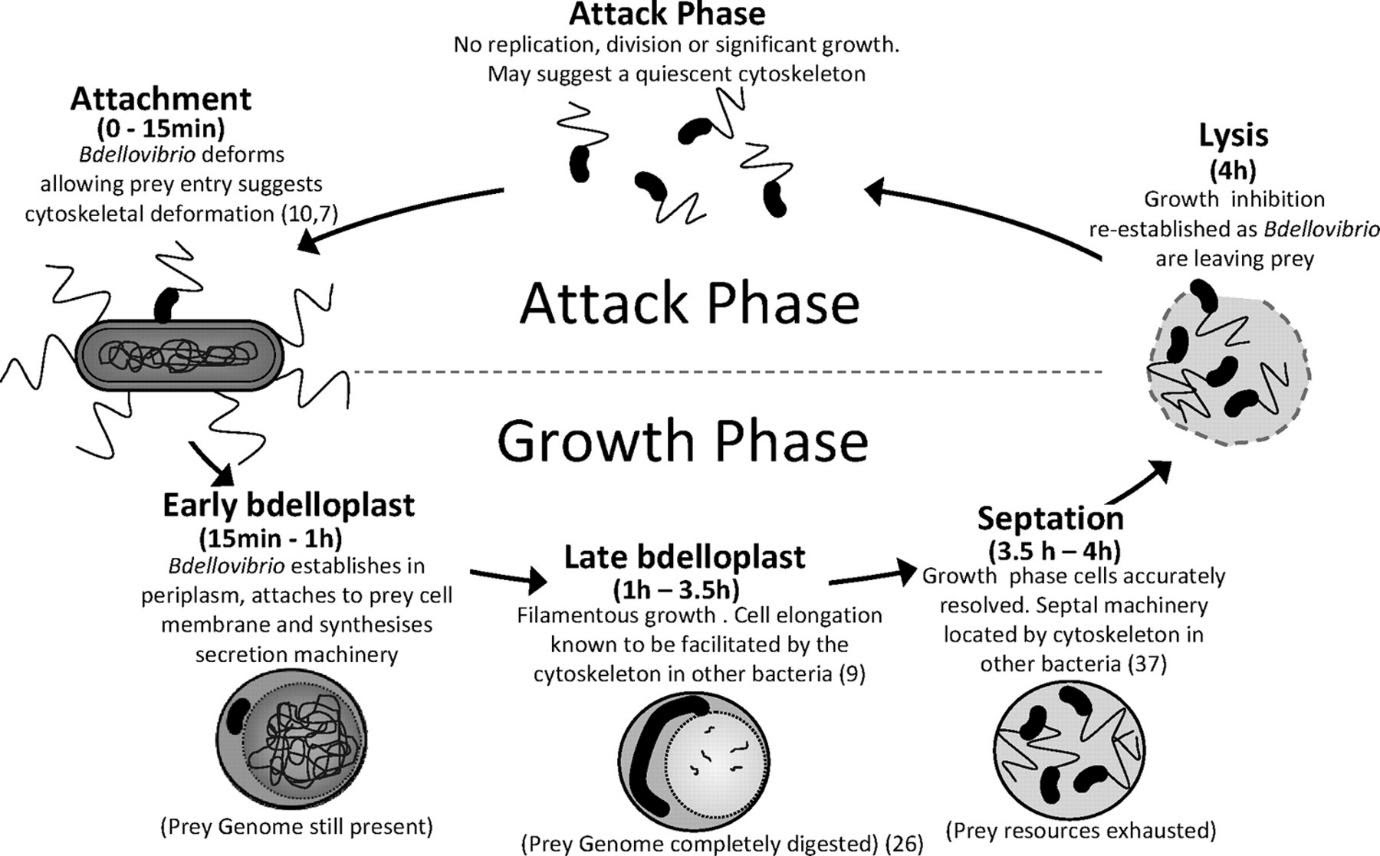
Figure: Life Cycle of Bdellovibrio bacteriovorus, a Gram-negative predatory bacterium
WHY IT IS THE BEST SOLUTION
Currently, there is great substantiation in support of the theory of using Bdellovibrio as a living antibiotic. Studies have shown that this Gram-negative predator kills other Gram-negative pathogens as prey, including some of the threatening ESKAPE pathogens, a group of clinically significant species that have developed multiple drug resistance and comprise of Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species. Furthermore, Bdellovibrio is also demonstrated to reduce the formation of biofilms in several Gram-positive and Gram-negative bacteria, via the release of enzymes. Additionally, these predators do not elicit an immune response from the body when ingested, injected or used topically, which is suggestive of their use against a wide range of Gram-negative pathogens. It is also very unlikely that the prey develops resistance to Bdellovibrio due to the fact that Bdellovibrio is non-antibiotic. Scientists have also stated that there is no transfer of genetic material between prey and pathogen which improves the likelihood of its safe use in the future.
LIMITATIONS AND FUTURE PERSPECTIVES
Despite its numerous advantages, one major drawback is that this predator is ineffective against Gram-positive organisms due to the thick peptidoglycan layer on the membrane of Gram-positives. Research, however, is trying to counteract this problem via combination approaches by amalgamating both predators and antibiotics in order to combat mixed bacterial infections caused due to both Gram-positive and Gram-negative pathogens. Another challenge that the scientific community is now facing is the sub-optimal effectiveness of Bdellovibrio in human serum due to high serum osmolarity. Although this snag is yet to be resolved, further ethical questions regarding the use of live bacteria in humans arise, which pose debatable scenarios. While the issue of acceptance can be settled with awareness, there are still several avenues unexplored pertaining to the use of predatory bacteria as a living antibiotic, making it a dynamic area of study for scientists. Considering the current situation revolving around drug resistance and emergence of superbugs as well as the existing treatment regimens, the use of predatory bacteria appears to be a sound and reliable solution for the post-antibiotic epoch.
Anagha S Setlur Master of Research graduate from Nottingham, UK,Molecular Microbiology. An avid reader of books and scientific publications with a history of working in several research projects in academia.