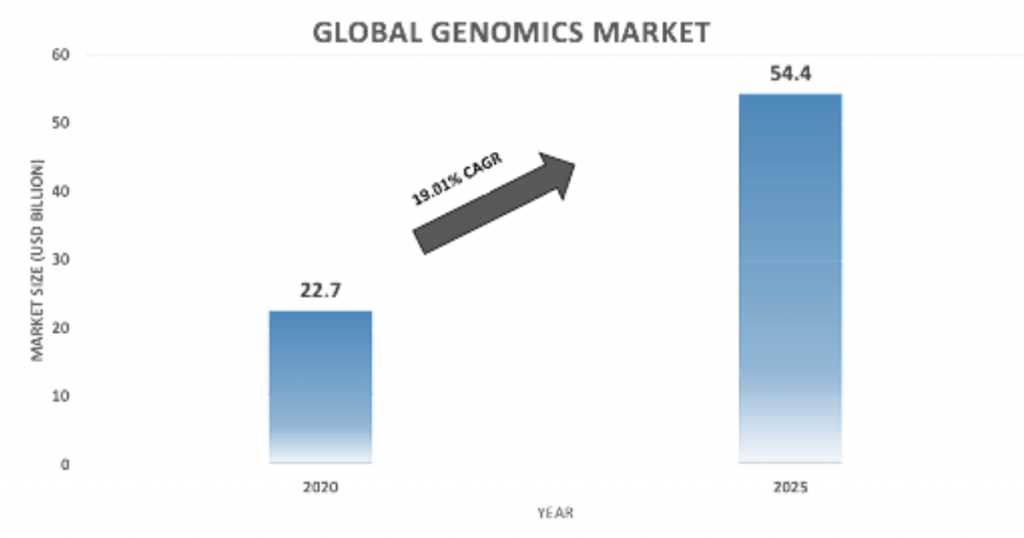
The burden of disease is escalating with every passing day and the inculcation of new technologies is promulgated for the diagnosis and treatment of various diseases. Genomic technologies are witnessing high demand from pharmaceutical and biotechnology companies owing to the increasing number of genetic research studies. The global genomics market is projected to reach USD 54.4 billion by 2025 from USD 22.7 billion in 2020, at a CAGR of 19.0% during the forecast period of 2021-2025.
Genomic sequencing is rapidly transitioning into clinical practice, and implemented into healthcare systems with support of substantial government investment, totalling over USD 4 billion, in at least 14 countries. Because of the rise of COVID-19 illness, the genomics market has gained steam thanks to huge government backing in 2021. Industrial and government sectors are working closely in coordination and are investing in advanced research projects to monitor the genome sequence of the novel coronavirus disease to develop COVID- 19 vaccines. From conventional treatment methods, the healthcare environment is gradually shifting towards newer technologies such as precision medicine, CRISPR etc.
The market has witnessed multiple product launches in 2020 thanks to an increase in competition for the event of genomic testing solutions for Coronavirus-19 (COVID-19).
Global Opportunities
The acceptance of genome editing/genome engineering in genomics, which is utilised for cell line engineering, genetic engineering, diagnostics, and medicines, is a major driver. Genome editing, for example, is used in plant target gene modification and the eradication of vector-borne diseases such as yellow fever, dengue, Zika, West Nile, Schistosomiasis, Leishmaniasis, and Lymes disease. The solutions to the removal of these diseases attract a significant amount of investment in the agricultural and healthcare sectors.
The current global pandemic has created considerable opportunities for the domestic market players operating within the space. The market has witnessed multiple product launches in 2020 thanks to an increase in competition for the event of genomic testing solutions for Coronavirus-19 (COVID-19). Manufacturers operating during this market witnessed high rates as health and well-being continues to get major attention in COVID-19 pandemic.
Global Market Challenges
Data from genomics can be used for a variety of purposes, including pharmacogenomics, diagnostics, and toxicogenomics. However, the analysis of huge volumes of data generated in genomics research is a major challenge. It is vital to recruit skilled professionals to analyse and interpret the outcomes of genome sequencing data due to the intricacies involved and the requirement for in-depth understanding in the field of genomics. But the major challenge is the shortage of trained professionals. There are very selective courses for genomics worldwide. In many countries there is no separate education program designed for the training of medical genetic professionals. The other challenge is security of Genomics data.
Many research suggest that providing Direct to Consumer (DTC) genetic testing could jeopardise privacy and data security, perhaps causing societal harm to consumers.
Genomics market has data privacy breaches so in some countries there are stringent regulations to enter the market. Organizations find it tough to enter because each country has its own set of regulations.
CRISPR technology has the potential to be a simple yet effective genome editing tool. It allows scientists to easily alter DNA sequences and gene functions.
New Technologies in use
Polymerase chain reaction (PCR)
This is one of the most used technologies. Polymerase chain reaction (PCR) is a laboratory technique which is used to amplify DNA sequences. The method involves using short DNA sequences called primers to select the portion of the genome to be amplified. One of the most extensively used diagnostic techniques for detecting pathogens, particularly viruses, that cause diseases including Ebola, African swine fever and foot-and-mouth disease is polymerase chain reaction (PCR). This technology is widely used during the COVID-19 pandemic. Small amounts of RNA from specimens are amplified into deoxyribonucleic acid (DNA), which is duplicated until Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is detectable if present, using PCR technique. Since its approval in February 2020, the PCR test has been the gold standard for diagnosing COVID-19. It’s precise and dependable.
Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)
“CRISPR” represents groups of short palindrome repeats at regular intervals. It has a unique DNA region with two distinct characteristics: a repeating nucleotide sequence and a spacer. The repeating nucleotide sequence, a component of DNA, is distributed throughout the CRISPR. The spacer is sandwiched between DNA fragments between these repetitive sequences.
CRISPR technology has the potential to be a simple yet effective genome editing tool. It allows scientists to easily alter DNA sequences and gene functions. It has a wide range of possible applications, including the correction of genetic abnormalities, disease therapy, disease prevention and the improvement of crops. CRISPR technology is based on bacteria and archaea’s innate defence mechanisms (in the field of single-cell microorganisms). To protect themselves from viruses and other external objects, these organisms use CRISPR-derived RNA and multiple CRISPR associated (Cas) proteins (including Cas9). They mostly work by severing and destroying the DNA of alien invaders. Genetic alteration or “editing” is permitted when these components are transferred to other, more complicated species.
CRISPR technology has been successfully employed to generate quick diagnostic tests for COVID-19, obtaining its first FDA approval (MD, USA) in the process. Multiple companies are racing to fill the ever-widening gap in the market caused by reagents for PCR-based COVID-19 tests running out and shrinking testing capacity as research continues for such tests to be developed for wider clinical use. Scientists have looked to CRISPR as a potential therapy in other areas, using its tailored enzymatic activity to break SARS-CoV-2 RNA and limit viral replication.
Precision medicine is an emerging approach for disease treatment and prevention that takes into account individual variability in genes, environment, and lifestyle for each person.
Precision Medicine
Precision medicine is an emerging approach for disease treatment and prevention that takes into account individual variability in genes, environment, and lifestyle for each person. This approach will allow doctors and researchers to predict more accurately which treatment and prevention strategies for a particular disease will work in which groups of people. For example, a person who needs a blood transfusion is not given blood from a randomly selected donor; instead, the donor’s blood type is matched to the recipient to reduce the risk of complications.
During the pandemic precision medicine has demonstrated to be effective in treating a variety of other ailments. Researchers created the Human Salivary Proteome Wiki in a recent study, which allows them to evaluate saliva samples for symptoms of infectious viruses.
Initiatives taken by Government of India
On January 3, 2020, the Department of Biotechnology (DBT) launched the ambitious “Genome India Project” (GIP). The goal of the GIP is to collect 10,000 genetic samples from Indian residents in order to create a reference genome. This programme represents India’s success in gene treatments and precision medicine, as well as its shift toward next-generation medicine, which allows for more customisation, safety, and early diagnosis. This programme would help establish the groundwork for individualised healthcare for a huge portion of the world’s population.
This project is coordinated by the Indian Institute of Science’s Centre for Brain Research in Bengaluru, which serves as the central coordinator for a cooperation of 20 premier institutions, each of which collects samples and conducts its own research. The Indian Institute of Science (IISc) in Bengaluru, as well as various Indian Institutes of Technology (IIT) are among the institutions engaged. For the study, data will be collected from participants via a simple blood test, and the information will be stored in biobanks by investigators in hospitals. This programme represents India’s success in gene treatments and precision medicine, as well as its shift toward next-generation medicine, which allows for more customisation, safety, and early diagnosis. This programme would help establish the groundwork for individualised healthcare for a huge portion of the world’s population.New technologies are coming up day by day and with government efforts and investment. The Genomics market will be one of the leading markets in the near future.
Composed by : “Dr. Ayushi Chaturvedi has done her graduation in dentistry and is currently pursuing PGDHM in Healthcare IT from IIHMR Delhi.Her areas of interest are: Health Informatics, Business Analytics and consultancy. She has also worked as an HR volunteer in Udayan Care,New Delhi.”
“Ms. Nikita Sabherwal is Healthcare Quality and Hospital Administration Professional.She is Associate Professor & Associate Dean (Training) in IIHMR Delhi. Ms. Sabherwal has worked with various top corporate chains of healthcare. She is also an empanelled NABH assessor and JCI trained.”
Photo by Unsplash

